Biotech stocks are often among the market’s most volatile, and Harpoon Therapeutics Inc. (NASDAQ: HARP) offers a perfect example of that.
The stock rocketed to an eight-month high Monday, advancing as much as 191% intraday before settling down to a gain of 22.61%. Shares closed at $1.41 Monday, up $0.26. Tuesday, the stock closed lower after gapping down at the open.
Monday’s huge upside action followed the company’s release of updated interim results for its myeloma drug that’s in Phase 1 clinical trials. The treatment is currently known as HPN217.
According to Harpoon’s news release, “The interim results, as of the data cut-off date of October 17, 2022, showed that HPN217 demonstrated continued evidence of clinical activity and a tolerable safety profile in heavily pre-treated patients” with relapsed/refractory multiple myeloma.
Relapsed/refractory multiple myeloma occurs when a patient has received treatment for a cancer called multiple myeloma, a rare cancer that affects bone marrow. It’s currently incurable, with the most common path being remission interspersed with periods when the disease becomes active. It’s termed relapsed/refractory multiple myeloma when the patient’s condition is especially difficult to treat.
Meaningful Clinical Benefits
“The encouraging initial clinical activity with deepening and durable responses observed in patients who have received multiple prior lines of therapy, combined with a generally well-tolerated safety profile, suggest the investigational T cell engager HPN217 may offer meaningful clinical benefits for patients with relapsed/refractory multiple myeloma,” said Al-Ola A. Abdallah, a doctor at the University of Kansas Medical Center, a principal investigator in the study.
“I look forward to continuing to study this promising drug candidate in these patients with advanced disease for whom there remains a significant unmet need for new treatment options,” Abdallah added.
Remarks like those gave investors hope.
San Francisco-based Harpoon went public in February 2019 at $14. The stock essentially meandered sideways until June of last year, when shares began declining.
On a rolling one-week basis, Harpoon is up 95.67%, although shares reversed lower in Tuesday’s season.
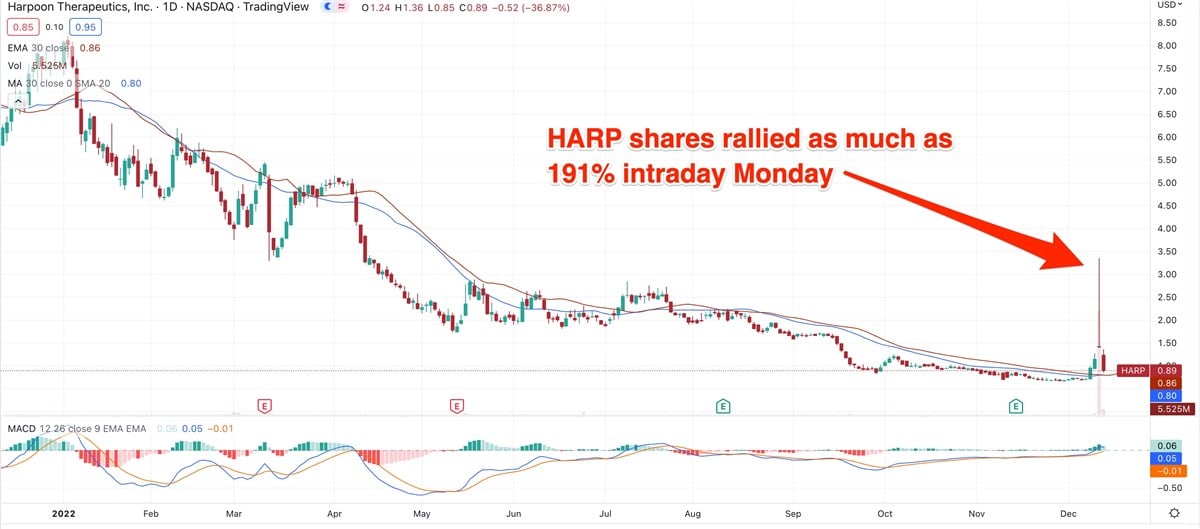
That type of action suggests quick profit-taking in the face of clinical-trial news that doesn’t directly lead to the monetization of a drug.
That’s especially true with a small company like Harpoon, whose market cap is just $31.1 million. Consistent with a company this size, Harpoon’s beta is 1.72, meaning it’s 172% more volatile than the broader market, as measured by the S&P 500 over a 12-month period.
Of course, a stock with a market cap of $31.1 million isn’t directly comparable to an index of the largest U.S. companies, but that still offers an indication of Harpoon’s propensity toward volatility.
Industry Home To Top Performers
The biotech industry is home to several large, well-established companies, including Amgen Inc. (NASDAQ: AMGN), Gilead Sciences Inc. (NASDAQ: GILD), Regeneron Pharmaceuticals Inc. (NASDAQ: REGN), Vertex Pharmaceuticals Incorporated (NASDAQ: VRTX) and Moderna Inc. (NASDAQ: MRNA). All are components of the S&P 500, and all are outperforming their index.
With many smaller companies within the industry also boasting market-beating performance, the group as a whole is outpacing most others. Pharmaceutical wholesalers and medical device makers are also showing higher returns than the broader market in recent months.
Harpoon remains a speculative stock. It’s often the case that biotechs become attractive acquisition targets if they have a treatment that a larger company wants to market. That could well be the case with Harpoon, although buying a stock with that strategy in mind is risky, as an investor may incur a large opportunity cost while waiting.
Harpoon has never been profitable, and analysts don’t see that changing anytime soon, although losses are narrowing and estimates have been revised higher recently.
In November, the company announced a corporate restructuring designed to slash operating expenses and better focus on clinical programs, including HPN217, that are expected to drive long-term growth.
Revenue for the most recent quarter came in $13.6 million, up 204% from the year earlier. The company attributed the increase to research and development services performed in a collaborative agreement with AbbVie Inc. (NYSE: ABBV).





